Service offered | In-silico functional inspection of implants
Fraunhofer IPA
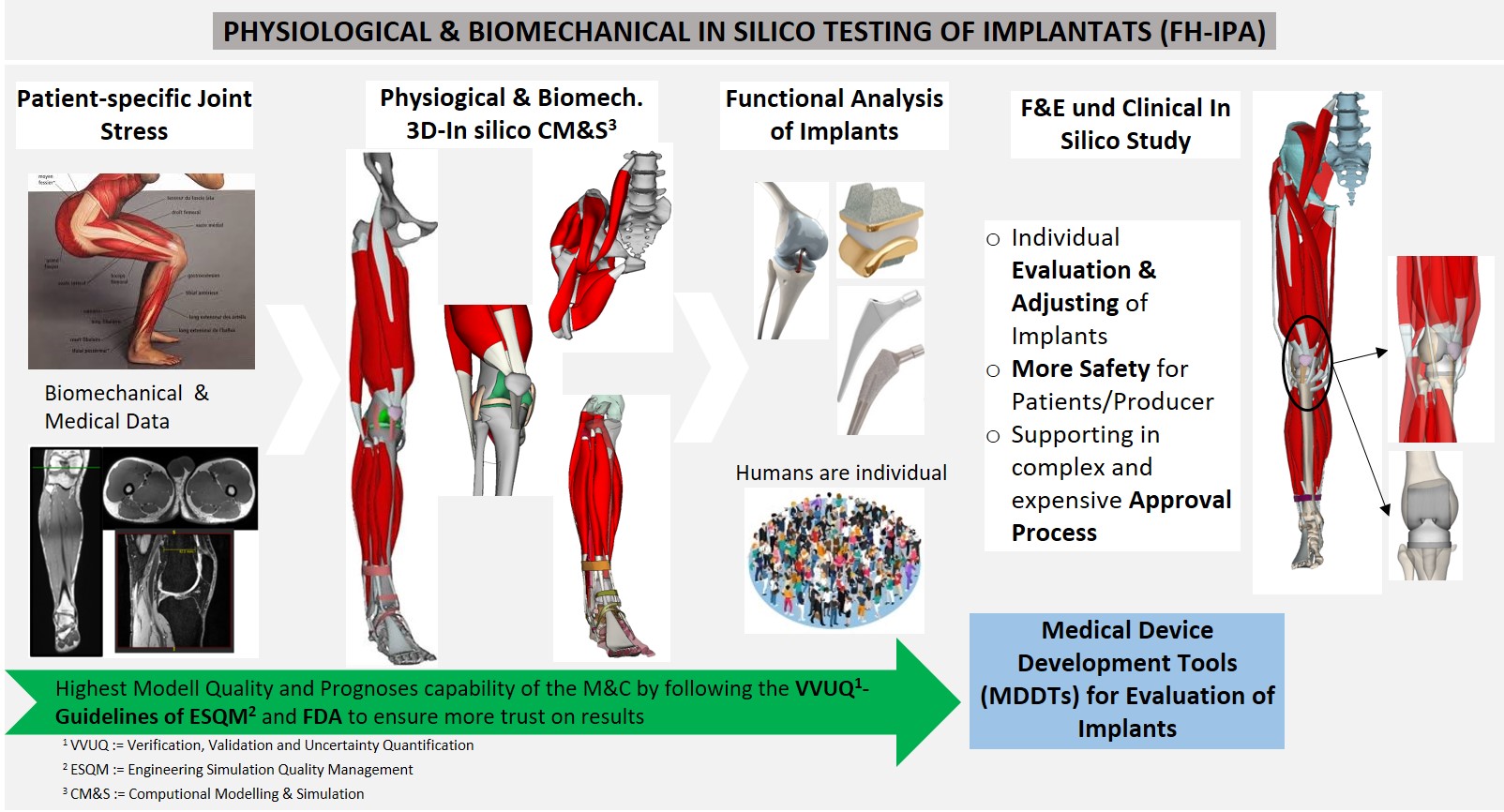
In-silico functional inspection of implants
At present, it is not possible to conduct patient-specific analyses of the function and effectiveness of implants in clinical trials. However, there is a solution to this problem in the form of verified and validated (V&V) in-silico analyses of patients together with the implant. This requires smart virtual processes from medical image capture and analysis, as well as biomechanical experiments, in-silico human simulations and CAE (computer-aided engineering) for products. Companies do not currently have these options available, however. An in-silico implant analysis on a large number of patients (> 100) in compliance with regulations can be carried out under realistic physiological and biomechanical conditions at a fraction of the time and cost associated with clinical implant trials. Another advantage of in-silico studies is that new and detailed insights into dynamic interactions between the implant and patient can be obtained from the complex simulations.
Services
- Biomechanical and structural-mechanical in-silico functional inspection of implants
- Biomechanical (dynamic) analysis and optimization of implant positioning in joints
- Generation of detailed musculoskeletal CAD-FEM simulation models from patient data (MRI, ultrasound)
- 3D FEM simulations of physiological joint systems (muscles, tendons and all connective tissue structures) with realistic kinematics
- Development of supporting biomechanical simulation and analysis processes for orthopedic surgery
- Further training in simulation technology for biomechanics and structural mechanics
- Development of simulation processes for personalized products
 Center Mass Personalization
Center Mass Personalization