The medical engineering sector in Germany is under constant pressure to innovate and stay compliant with regulations. Comprising numerous distributors and suppliers, it generates sales in the tens to hundreds of billions across the country. However, the successful use of simulation methods to support approval processes remains a challenge. We can help you take your innovative strength to the next level.
Goals
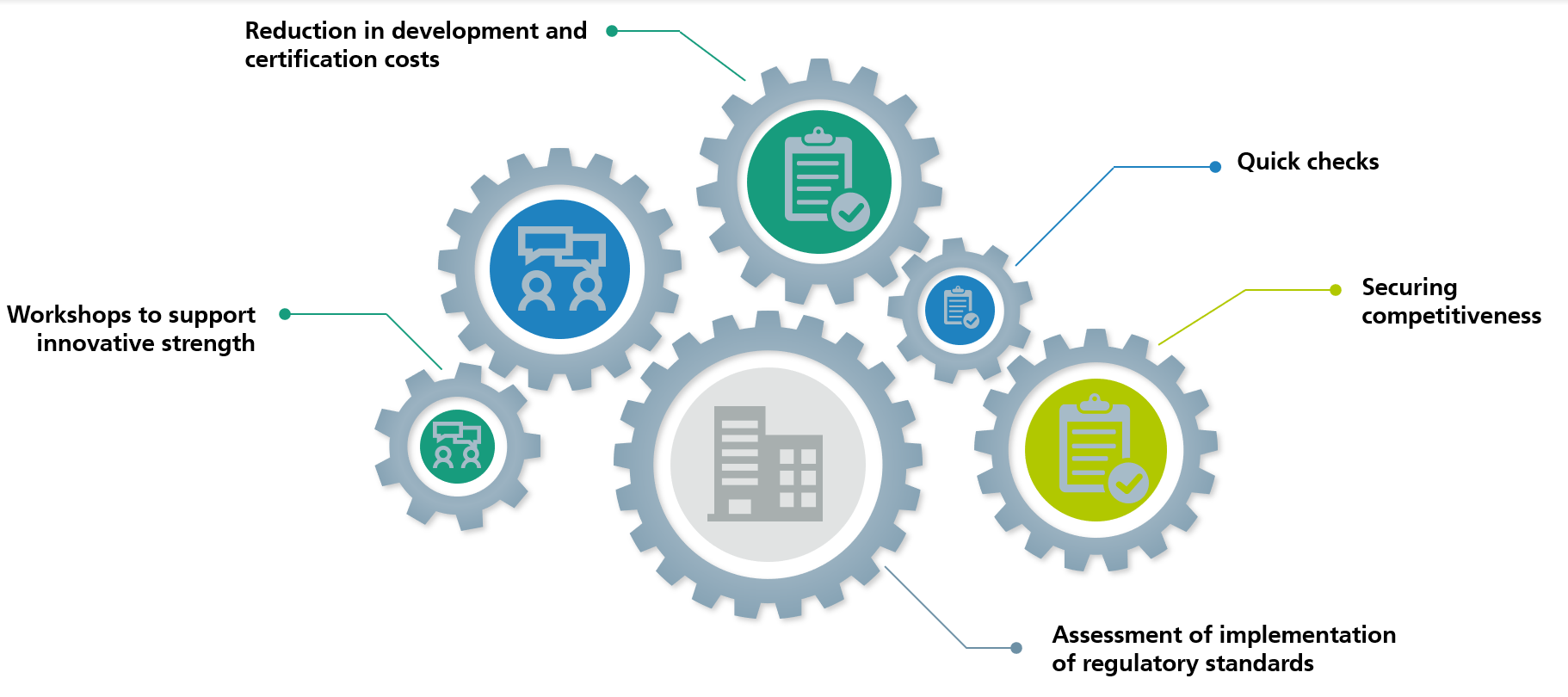
- To reinforce innovative strength: reinforcing innovative strength in your company by identifying potential and process improvements based on simulation methods
- To assess how regulatory standards are implemented: performing gap analyses for regulations and implementation
- To reduce costs: reducing development and certification costs for medical devices by introducing in-silico processes with virtual patient cohorts
- To support international competitiveness: securing global competitiveness by providing support for the development and implementation of essential basic technologies
Services offered
Our services are focused on supporting the innovative strength of German medical engineering companies by introducing simulation methods to optimize processes, ensuring compliance with regulatory standards, reducing costs and supporting global competitiveness through the implementation of essential basic technologies.
If you have any questions, please get in touch — we look forward to discussing your needs.
 Center Mass Personalization
Center Mass Personalization